Journal of Applied Biosciences 212: 22504 – 22517
ISSN 1997-5902
Coupling Optimized Plasma/Coagulation-Flocculation Processes to Cactus Extracts in a Water Treatment Approach polluted by Ridomil – Gold 66WP Plus.
Tadom Doringar1, Mbailaou Goussou2, Kamgang Youbi Georges3, Laminsi Samuel4
Department of Chemistry, Adam Barka University of Abéché, P.O. Box : 1173 Abéché, Chad.
Department of Chemistry, University of Sarh, P.O. Box: 105 Sarh, Chad.
Department of Inorganic Chemistry, University of Yaoundé I, P.O. Box 812, Yaoundé, Cameroon
Author for the correspondence: tadomdoringar@gmail.com.
Submitted 01/08/2025, Published online on 30/09/2025 in the https://www.m.elewa.org/journals/journal-of-applied-biosciences https://doi.org/10.35759/JABs.212.7
ABSTRACT
Objective: to improve the treatment of the synthetic solution of Ridomil-Gold 66WP Plus (100mg/L) at unadjusted pH (6.9) with Aluminum Sulfate (Al2(SO4)3.18H2O) and Iron (III) Sulphate Fe2(SO4)3.7H2O assisted by cactus extract adjuvant (natural flocculant). This improvement is based on an optimization of the coagulant quantity and pH of the Ridomil – Gold 66WP Plus solution, which is essential for easy coupling.
Methodology and results: On this basis, to conduct a study to establish the efficacy of our new flocculant based on the results obtained, in order to demonstrate that the cactus extract is able to improve the coagulation/flocculation process of effluents containing pesticides, coupled with the pre-treatment of the plasma process for the reduction of the volume of sludge formed. Indeed, the exploration of this optimal condition leads to the abatement percentages of Ridomil-Gold 66WP Plus approximately 100%, the abatement of Turbidity (90%), Chemical Oxygen Demand (80%) for the combination of cactus extract with Aluminum and Iron (III) sulfate.
Conclusion and application of the results: Although these treatments (plasma coupled with coagulation-flocculation) have proven to be very effective, they are longer, using additives (coagulants and flocculants) that produce sludge that varies from 1.28 to 57.86% which is unfortunately a source of secondary pollution. The plasma/coagulation coupling optimizes the treatment by increasing the abatement rate of approximately 100% of the Ridomil-Gold 66WP Plus, with a reasonable coagulant and flocculant consumption and a low sludge production of 7.5%. These results make it possible to use the bioflocculant extracted from cacti in water treatment by coagulation/flocculation with a view to replacing flocculants and possibly chemical coagulants likely to induce harmful effects on human health. In addition, this coupling also reduces the energy costs associated with higher mineralization of wastewater by non-thermal plasma processes.
Keywords : Ridomil-Gold 66WP Plus, Cactus Extract, Plasma/Coagulation-Flocculation.
INTRODUCTION
Source of water pollution from organic pollutants such as pesticides poses enormous risks to human and animals health. In addition to drift and spillage of residual spray solutions or rinse water from sprayed water courses, persistent pesticide residues in soils can be transported to surface waters through run off and to ground water through drainage (Gouda et al., 2018). These pesticides exist in a variety of forms, both natural and anthropogenic, but those known for their persistence and bioaccumulation constitute a large number. Before the advent of pesticides, cropping systems were designed to ensure the best compromise between pest risk and crop production potential (Lauri, 2012). Gradually, the acquisition of knowledge about the mineral requirements of a crop and the control of fertilization have led to the establishment of a pesticide production system to eliminate competition from weeds. During its land journey, raw water systematically leaches the land and is loaded, in varying quantities and proportions depending on the location, with high concentrations of pesticides. All these pesticides and the products of their subsequent degradation or transformation in the environment are present in various physical states, either still in solution or adsorbed to soil particles suspended in water (Dihang, 2007). Water that reaches the water table by percolation carries with it loads of contaminants whose concentrations in solution can be toxic to aquatic fauna and flora and make the water unfit for human consumption if the drainage network is a source of abstraction for drinking water. The systematic use of these products is being questioned, with the growing awareness of the risks they can generate for the environment and human health. The pesticide used for this work is Ridomil – Gold 66WP Plus widely used as an antifungal agent, mainly in tropical areas (Monkiedje et al., 2007). It is a fungicide used to protect plants from pests such as phytophthora SP., which causes black pods. For agricultural effluents contaminated with Ridomil-Gold 66WP-Plus, the literature describes removal techniques that consist of conventional and advanced oxidation like adsorption, coagulation/flocculation, electrocoagulation, advanced oxidation processes such as plasma (Glidarc) and precipitation (Suciu et al., 2011; Al Hattab and Ghaly, 2012; Pliego et al., 2014; Ahammed Shabeer et al., 2015; John et al., 2016). The coupling of the sliding discharge of moist air (Glidarc) to coagulation/flocculation aims to reduce the pollutant load before proceeding with coagulation/flocculation, to reduce the volume of sludge produced in large quantities during coagulation alone. In addition, the use of cactus extract as a bioflocculant is part of a strategy for the sustainable development of the environment, we were interested in the valorization of a new biodegradable natural product, as a flocculant in the physico-chemical treatment process with a view to using it as a non-toxic natural flocculant likely to replace chemical flocculants harmful to human health (Abid et al., 2009).
MATERIALS AND METHODS
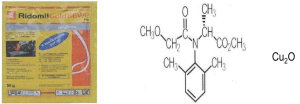
Synthetic effluent: The effluent chosen for this work is a synthetic pesticide-fungicide solution marketed under the name Ridomil-Gold 66WP Plus marketed by the company Riedel Haen and used at a high purity. In systematic nomenclature, it is (methyl (2,6-dimethyl-phenyl)–N-methoxiacetyl) or Metalaxyl-M or Mefenoxam its crude formula is C15H21NO4 of molar mass 279.3g/mol. It is an organic compound consisting of 64.47% C; 7.52% of men; 0.36% N and 23% O associated with copper(I) oxide (Cu2O) which plays the role of antifungal. It is soluble in water at 25°C at 26g/L at pH = 7 (PMRA 2007).
| Cu2O |
Figure 1 : 50g packaging of Ridomil Go/d 66WP Plus product Chemical structure of Ridomil-Gold 66WP Plus (methyl (2,6–dimethyl-phenyl) – N-methoxiacetyl) and copper oxide I
The synthetic effluent of the fungicide was prepared by dissolving Ridomil-Gold 66WP Plus powder in distilled water. The solution of the Ridomil-Gold 66WP Plus has a maximum absorption wavelength of 498 nm. The pH of the samples was adjusted by solutions of H2SO4 at 1M and NaOH at 1M. The Chemical Oxygen Demand (COD), Turbidity, Absorbance (at 480nm), pH and Total Organic Carbon (TOC) of the initial solution (100mg/L) were: 480 mg (of O2)/L; NTU 399.66; 0,231; 6,9 ; 297.5mg/L respectively.
Cactus leaves: The cactus leaves, the extract of which was used as a flocculant in the treatment of the Ridomil-Gold solution, is a food plant of the cactaceae family (Soulaire, 1997), an erect, branchy, xerophilous tree-bearing plant native to Mexico (Russel, Felker, 1987) 2 to 6 meters high.
Figure 2 : Cactus plant (opuntia Tuna) used as a natural flocculant for the treatment of Ridomil-Gold 66WP Plus.
It has very appreciable therapeutic, nourishing and cosmetic properties. It is recommended for diabetics with insulin-independent diabetes because their consumption can improve sugar control in patients and can reduce cholesterol levels in the blood (Frati et al., 1988; Fernandez, et al., 1990). It also has a diuretic effect and antiviral and antitoxic properties compared to the insecticide Chlorpyrifos (Florian C Stintzing et al., 2005) and as a remedy for prostate dysfunction (Pimienta-Barrios, 1993 and 1994). It also has an antiglycemic slimming effect, rich in vitamin C similar to that of oranges, apples, pears, apricots, cherries, etc. (Sepulveda and Saenz, 1990; Barbera et al., 1992) β-carotene, which is a precursor to vitamin A (Rodriguez and Cantwell, 1988). Racket mucilage is used in the manufacture of shampoos, hair softeners, dermal creams and moisturizing milks (Pimienta Barrios, 1994).
Cactus Extract: These leaves were collected, cleaned, washed with water and crushed. The aqueous extract obtained after grinding was diluted to 10% in distilled water and then homogenized by stirring for 30 minutes. The cactus extract is obtained by filtration after 30 minutes of decanting. The solution obtained is relatively stable and would be preserved in only one day; after this time, the cells would enter a state of decomposition and lose 85% of their viscosity (Heredia et al., 1988).
| Crushed cactus leaves |
| Cactus Leaves |
| Cactus leaf extract |
Figure 2: Cactus juice extraction process
It is a viscous liquid of green color miscible with water, its density is 1.008Kg/L of pH 6.5 and contains 98% water (Abid et al., 2009).
Coagulation-flocculation process: Two inorganic coagulants were used for this investigation: Al2(SO4)3.18H2O and Fe2(SO4)3 with some purity. The coagulation-flocculation experimental device is based on the use of Jar-test consisting of 6 (six) beakers of 500ml each containing 450mL of Ridomil-Gold 66WP Plus solution. The masses of the coagulants introduced into the reactor vary from 0.25; 0,5 ; 0,75 ; 1 ; 1.25, 1.5 and 1.75g the whole thing is subjected to a strong magnetic stirring of 800 rpm for 10 minutes. After adding 10mL of the cactus extract, we reduced the stirring speed to 40rpm for 3 minutes followed by adjusting the pH. After thirty minutes of settling, the supernatant of the solution was sampled, the absorbance of which is measured at the maximum length (λmax = 498nm) using an AQUALYTIC SPECTR-DIRECT spectrophotometer (200 – 800nm). The residual concentration of Ridomil (mg/L) in the supernatant is determined from the calibration curve. The percentage of Ridomil allowance is calculated using the following formula:
Abatt (%) = x 100 (1)
Where Abst is the residual absorbance after coagulation and Abs0 is the initial absorbance of the pesticide. Chemical oxygen demand (COD) was measured by the standard method using potassium dichromate, an oxidizing agent; Turbidity was measured by a HACH type LTD 082.99.40001 turbidity meter. The pH is measured by a HANNA HI 9811-5 pH meter with a glass electrode. Total Organic Carbon (TOC) is measured by using an analyzer equipped with a self-sample (ASI-5000) and a Platinum-based catalyst. The TOC (analyze) is calibrated by using a solution of potassium hydrogen phthalate. The sludge formed at the bottom of the reactor is collected and dried by evaporating water at 110°C and the volume is then measured. The percentage of sludge formed is calculated by the following formula:
Sludge (%) = x 100 (2)
Where VS is the amount of sludge formed in (mL), V0 is the initial volume of sludge in the effluent (mL).
Figure 3 : Diagram of the experimental Jar-test device used for the treatment.
Co (Ridomil) = 100mg/L, V= 500ml, pH= 6.9, T= 25°C
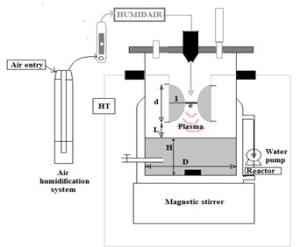
Non-thermal plasma pretreatment: The Glidarc used for pretreatment is a source of non-thermal plasma, the experimental design of which is schematized below (Fouodjouo et al., 2015; Njiki et al., 2016). Plasma gas is moist air ejected into the reactor along the divergent electrodes with a flow rate of 800L.h-1. The geometric parameters of the reactor and the production of plasma gas are presented in Figure 1 (Brisset et al., 2008; Moussa et al., 2007). To this end, the following reactions can be proposed (Benstaali et al. 2002, Brisset et al., 2008; Brisset et al. 2011; Brisset and Hnatiuc et al., 2012).
H2O + e– H• + OH• + e–
O2 + e– O (3P) + O(1D)
N2 + e– N(4S) + N(2D)
N(2D) + O2 NO• + O
H• + O2 HO2•
HO2• + NO• NO2 + HO•
NO2 + HO• H+ + NO3
NO• + HO• HNO2
HNO2 H+ + NO2–
NO• + O(3P) NO2
Figure 4: Glidarc experimental set-up: e = 3mm (inter-electrode distance), L= 1.5cm (solution-electrode interface), D = 8cm (reactor diameter), H = 10cm (solution height), d = 7cm (length of electrodes), I = 2cm (radius of electrode).
For non-thermal plasma pretreatment, 450 mL of Ridomil solution (100mg/L) introduced into the reactor and subjected to magnetic agitation, exposed to Glidarc plasma of moist air. This solution is maintained at a temperature of 25 ± 2°C by cooling water that circulates through the double wall of the reactor. The treatment times t* (5, 10, 15, 20, 25, 30, 40 and 45 minutes) and the residual concentrations of Ridomil are determined by using formula (1) in the previous paragraph.
Treatment by non-thermal plasma coupling/coagulation-flocculation:450 mL of Ridomil solution (100 mL) was pretreated with continuous discharge plasma to expose the solution directly to the plasma plume. Thus, the pre-treatment times of 20 minutes and 40 minutes were retained as optimal for the subsequent treatment (slaughter (%) ≥ 80). The solution of the pollutant pretreated by plasma was then used for the coagulation-flocculation treatment with two coagulants Fe2(SO4)3 and Al2(SO4)3 and the parameters (Turbidity, COD, TOC, Absorbance, Abatement Rate and Percentage of Sludge) were evaluated. Before this second coupling step, the pH of the plasma-pretreated solution is adjusted to 6 for the experiment with Fe2(SO4)3 and pH 5 for Al2(SO4)3. For these pH values, the effect of the post-discharge of the plasma that could occur was neutralized in the second step (Naïtali et al., 2010).
RESULTS AND DISCUSSIONS
Plasma pretreatment of Ridomil-Gold 66WP Plus
Monitoring the abatement rate: The variation in the absorbance of the aqueous solution of Ridomil-Gold 66WP Plus (100mg/L) at several exposures to Glidarc (5-minute increment time) is recorded, abatement rates are calculated. The results obtained after treatment with Glidarc are summarized in the figure below.
Figure 4: Abatement rate as a function of processing time.
There is a gradual increase in the rates of abatement of the pollutant as the duration of exposure to plasma increases. These abatement rates vary from 70.12 to 73.16% for processing times of 30 to 45 minutes. These results are consistent with those of previous studies of pesticide degradation by humid air plasma (Bi et al., 2013; Li et al., 2013, Fouodjouo et al., 2015). In chemical plasma treatment, the removal of pesticides is due to the primary HO• species created in the landfill and the formation of water-soluble secondary species such as H2O2, ONOO– (Brisset et al., 2008, Brisset and Hnatiuc 2012, Fouodjouo et al., 2013). However, after 45 minutes of treatment, the discoloration is not over. While in an advanced oxidation process, the main problem remains the increase in efficiency, and the decrease in exposure time aimed at reducing this cost of treatment, in coagulation/flocculation of the process, the main advantage is also the increase in efficiency without much sludge aimed at avoiding secondary pollution.
Absorbance monitoring: The experimental results illustrated in the figure below show that a minimum absorbance of 0.062 can be achieved after 45 minutes of treatment. This proves the degradation of the vast majority of polluting molecules and therefore shows the effectiveness of the Glidarc technique in treating and breaking down Ridomil-Gold 66WP Plus. Similar results have been obtained for the treatment of effluents polluted by organic compounds in aqueous environments (Doubla et al., 2007).
Figure 5: Evolution of the absorbance measured at 498nm of a 66WP-Plus Ridomil-Gold solution exposed to plasma
Coagulation-flocculation treatment
Case of coagulant Al2(SO4)3 . 18H2O
Influence of pH and coagulant dose on the reduction rate: Each test portion is performed on 500mL of Ridomil-Gold 66WP Plus solution at 100mL, coagulation is performed with doses ranging from 0.25 to 1.75g of aluminum sulfate followed by the adjusted pH and flocculation step per 10mL of the cactus extract.
Figure 6: Influence of pH and coagulant dose on the reduction rate: V=500mL,
C0 (Ridomil) = 100mL, V (cactus extract) = 10mL
According to the results presented in the figure below, it can be seen that the optimal doses of the coagulant vary from 0.5 to 1.75 g at pH 5, from 0.25 to 1.75 at pH 7, corresponding to the abatement yields which are about 100% for each of the two cases. In addition, the results obtained confirm the bibliographic data (Achour, 2003; Rezek, 2004) concerning the removal of organic molecules for pH values 5 and 7 depending on the nature and structure of the compound as well as the nature of the coagulant. We can also note better abatement rates at pH6 and 9 of 85.71 to 94.80% and from 95.57 to 98.70% respectively at the same doses of coagulants as in the previous cases. We can say that the removal efficiency has significantly improved at acidic (pH5), neutral (pH7) pH, followed by acid (pH6) and then basic (pH9) pH.
Case of coagulant Fe2(SO4)3. 7H2O
Influence of pH and coagulant dose on the reduction rate: We can observe that the optimum rate of abatement corresponds to pH 4 and 1.5g of dose of Iron (III) sulphate. This high abatement rate of 99.13% obtained is explained by the strong interaction between the hydrolyzed forms of Iron (III) and the suspended organic matter.
Figure 6: Influence of pH and coagulant dose on the reduction rate: V=500mL,
C0 (Ridomil) = 100mL, V (cactus extract) = 10mL.
On the other hand, other better abatement rates obtained at pH 5,6,7,9 at doses 1g and 1.75g, i.e. 96.96%, deserve to be noted. The use of cactus extract brings an improvement in abatement rates that has extended to pH 2.5 with 94.84% at 0.5g of coagulant dose. At the same pH, the abatement yield dropped from the added mass of 1.25g of Fe2(SO4)3 to 63.63%. This phenomenon is due to the distribution of Iron (III) ions linked to agitation during coagulation. At the other pH levels, the abatement rates are about 90%. In addition, the results obtained confirm the bibliographic data concerning the elimination of colloidal organic compounds for pH 5 to 7 depending on the nature of the coagulant (Carrier et al., 2007; Canizares, 2009).
Plasma/coagulation-flocculation coupling treatment: The results obtained during coagulation/flocculation treatments carried out by using the two coagulants Al2(SO4)3. 18H2O; Fe2(SO4)3.7H2O combined with cactus extract, made it possible to remove a good part of the pollutant load of Ridomil-Gold 66WP Plus. However, it generates a high quantity of physico-chemical sludge, mainly organic, which becomes a nuisance for the future of the treatment, which would have to be disposed of because of the toxicity of Ridomil-Gold 66WP Plus. 450mL of Ridomil-Gold 66WP Plus solution (100mg/L) was pretreated with continuous discharge plasma to expose the solution directly to the plasma plume, for t*= 20mn and 40mn. The pre-treated plasma pollutant solution was then used for coagulation/flocculation treatment with the two coagulants Al2(SO4)3 and Fe2(SO4)3 and the parameters (Turbidity, COD, TOC, Absorbance, abatement rate, percentage of sludge) were evaluated. Before this second coupling step, the pH of the plasma-pretreated solution is adjusted to pH 6 for the experiment with Fe2(SO4)3 and pH 5 for Al2(SO4)3. For these pH values, the effect of the post-discharge that could occur was neutralized in the second step (Naïtali et al., 2010).
Combination of Aluminum Sulfate/Cactus Extract and Iron (III)/Cactus Extract
Coupling to 20 min. plasma pretreatment
Table 1: Evidence of improvement during coupling (plasma/coagulation-flocculation)
| Coagulants | Parameters | No pretreatment
plasma |
With pre-treatment
plasma |
Variation |
| Al2(SO4)3 .18H2O
(0.25g) pH7 |
Abatt (%) | 100 | 100 | ̶ |
| DCO (%) | _81,04 | 97,21 | +2,9 | |
| Abatt de Turb (%) | 93,66 | 92,24 | -1,52 | |
| Volume builds (mL) | 37,1 | 7,5 | -79,78% | |
| Fe2(SO4)3.7H2O
(1,5) pH7 |
Abatt (%) | 100 | 100 | ̶ |
| DCO (%) | 94,37 | 97,83 | +3,54 | |
| Abatt de Turb (%) | 94,42 | 91,66 | -2,92 | |
| Sludge volume(mL) | 112,5 | 75 | -33,33% |
The use of 10mL of the new cactus extract product combined with 0.25g of Al2(SO4)3.18H2O at pH 7 and then 1.5g of Fe2(SO4)3.7H2O at pH 4 at coagulation/flocculation of Ridomil-Gold 66WP Plus pre-treated for 20 minutes in plasma quickly reached the maximum elimination rate. Both coagulants achieved high values of Turbidity and COD removal (>90%) except for the non-pretreated sample, the COD is about 80% in the case of Al2(SO4)3.18H2O. Under these optimal conditions of pollutant reduction, a reduction of 79.78% of sludge obtained with the coagulant Al2(SO4)3.18H2O while with the coagulant Fe2(SO4)3.7H2O reached the 33.33% reduction of sludge, which is not very advantageous compared to the previous case which is equivalent to double.
Coupling to the 40-minute plasma pretreatment
Table 2: Evidence of improvement during coupling (plasma/coagulation-flocculation)
| Coagulants | Parameters | No pretreatment
Plasma |
With pre-treatment
Plasma |
Variation |
| Al2(SO4)3.18H2O
(0,25g) pH 7 |
Abatt (%) | 100 | 94,4 | -5,6 |
| DCO (%) | 81,04 | 95,62 | +15,25 | |
| Abatt de Turb (%) | 93,66 | 97,22 | +3,66 | |
| Volume builds (mL) | 37,1 | 37,5 | +1,06% | |
| Fe2(SO4)3.7H2O
(1,5) pH 7 |
Abatt (%) | 100 | 87,44 | -12,56 |
| DCO (%) | 94,37 | 94,10 | -0,24 | |
| Abatt de Turb (%) | 94,42 | 95,23 | +0,81 | |
| Sludge volume(mL) | 112,5 | 37,5 | -75% |
Coagulation/flocculation treatment under the same prerequisites after a 40-minute plasma pretreatment of Ridomil-Gold 66WP Plus provided satisfactory results. These results are close to 97% turbidity removal and 95.62% COD for the coagulant Al2(SO4)3.18H2O, 95.23% and 94.10% respectively turbidity removal and COD for Fe2(SO4)3.7H2O. Unfortunately, there is an increase in volume of +1.06% for Al2(SO4)3.18H2O, which can be explained by the phenomenon of water evaporation from the synthetic solution due to the long treatment times which can make the following process unfavorable. Thus, it is significantly improved in the case of the coagulant Fe2(SO4)3.7H2O with a reduction of 75% smaller than the initial percentage.
CONCLUSION AND APPLICATION OF RESULTS
The use of plasma in combination with coagulation/flocculation was intended to improve the process. The aqueous solution of Ridomil-Gold 66WP Plus, was chosen as the model pollutant for the investigation. For the coagulation process, two classical coagulants were used: Al2(SO4)3.18H2O and Fe2(SO4)3.7H2O and it was shown that the coagulation process is not affected by the types of coagulants. Thus, the use of cactus extract as an adjuvant to flocculation provides better yields. It has also been noted that for the Al2(SO4)3/cactus extract combination, doses ranging from 0.25 g to 1.75 g at pH7 have a reduction rate of 100%. As a result, cactus extract combined with Al2(SO4)3 has the same efficacy as Fe2(SO4)3. Thus, the improvement of coagulation/flocculation efficiency in the treatment of pollutants is possible by combining coagulants with our new natural flocculant: cactus extract. This combination used for the treatment of pesticide-containing pollutants has given satisfactory results and proves that cactus extract can be a valid substitute for industrial flocculants. Coagulation/flocculation has led to the production of sludge which is embarrassing as far as the environmental aspect is concerned. Combined treatment increases the efficiency of water treatment: the total elimination of pollutants is obtained, resulting in a reduction in COD, turbidity (> 90%). In addition, the combined use of plasma and coagulant resulted in the production of sludge volume with a 75% reduction in the amount produced than when the coagulant alone was used for treatment. This demonstrates that there is a positive synergy between coagulation/flocculation and non-thermal sliding discharge plasma. This combination of processes used for the treatment of pollutants containing pesticides has given a satisfactory result and deserves to be brought to light for researchers who are looking for innovation to make this process, which until now has been described as classic, more and more efficient.
REFERENCES
Abid, A., Zouhri, A., Idec, A, & Kholtei, S. (2009). Valorization of a new bioflocculant (cactus extract) in the physico-chemical treatment of liquid discharges loaded with copper, zinc suspended solids. RER (Revue des Energies Rénouvelables). Vol. 12 No. 2. 321-330.
Achour, S. (2003). Impact of Chlorination, Flocculation and Adsorption Processes on the Evolution of Organic and Mineral Compounds in Natural Waters, State Doctoral Thesis, University of Tizi-Ouzou, Algeria.
Ahammed, S.T.P., Saha, A., Gajbhieye, V.T., Gupta, S., Manjaiah, K.M., & Varghèse.E. (2015). Exploitation of nano-bentonite, nano-halloysite and organically modified nano montmorillonite as a adsorbed and coagulation aid for the removal of multi pesticide from water; a sorption modelling. Water Air and soil pollution, 22C, 41. https//doi.org/10.1007/s 11270-015-2331-8.
Al Hattab, M. T., & Ghaly, A. E. (2012). Disposal and treatment méthods for pesticides containing wastewater: critical rewiew and comparative analysis. JEP (Journal of Environmenta Protection), 3, 431-453.
PMRA (Health Canada’s Pest Management Regulatory Agency) (2007). Proposed Re-evaluation Decision PRVD2007-10 Metalaxyl and Metaaxyl-M, 34P.
Barbera, G., Carimi, F., and Inglese, P., 1992. Paste and present role of the Indian prickly pear (Opuntia ficus indica(L) Mill., Cactacées) in the agriculture of Sicily. Economic botanic, 46(1), p 1020.
Benstaali, B., Boubert, P., Cheron, B. G., Addou, A. & Brisset, J.L. (2002). Density and rotational temperature measurement of the NO and OH radicals produced by a glyding arc in humid air and their interaction with aqueous solution.
Brisset, J.L., & Hnatiuc, E, (2012). Peroxynitrite: a reexamination of the Chemical properties of non- thermal discharges burning in air over aqueous solution. Plasma Processing, 32, 655-674.
Brisset, J.L., Benstaali, B., Moussa, D., Fanmoe, J., & Njoyim-Tamungang, E. (2011). Acidity control of Plasma-Chemical oxidation: application to dye removal, urban waste abatment and microbial inactivation. Plasma Sources Sciences and technology, 20, 034021(12pp).
Brisset, J.L., Moussa, D., Doubla, A., Hnatiuc, E., Hnatiuc, B., Kamgang Youbi, G., Herry, J.M., Naïtali, M., & Bellon-Fontaine, M. N. (2008). Chémical reactivity of discharge and temporal post- discharges in plasma treatment of aqueous media: example of gliding dischages treated solutions. Industrial and Engineering Chemistry Research, 47, 5761-5781.
CEC (1976). Framework Directive of CS 76/464/EEC of 4 May 1976 on pollution caused by certain dangerous substances discharged into the aquatic environment of the European Community. Official Journal of the European Communities, No L 129 of 18 May 1976.
CEC (1980). Directorate of CS 80/778/EEC of 15 July 1980 – On the quality of water intended for human consumption. Official Journal of the European Communities, No L 229/11 of 30 August 1980.
Dihang, M. D. (2007). Coagulation and flocculation mechanism of dilute clay suspension, encountered in water, Doctoral Thesis, Paul Sabatier University, Toulouse (III), France.
Fernadez, M.L., Treya, A., and Me Namara, D.J., (1990). Pectin isolated from prickly pear (Opuntia Sp) modifies low density lipoprotein metabolism in Cholesterol-field guinea pigs. J. Nutr., 12, Vol:1280-1290.
Florian, C. S., Kirsten, M. H., Markers, R. M., Reinhold, C., Weiguang, Y., Subramani, S., Casimir, C. A., Ron, Bunch., Pierre, F., 2005. Color, betaine pattern and antioxidant properties of prickly pear (Opuntia Spp) clones. J Agric Food Chem. 26 Jan; 53(2) : 442-5. doi:10.1021/JF040751Y.
Fouodjouo, M., Laminsi, S., Kamgang-Youbi, G., Mengue, M.T.& Debacher, N.A. (2015). Non-thermal plasma induced total mineralization of glyphosate in water in the presence of iron II. Journal of Brazilian Chemical Society, 26, 411-419.
Frati, A. C., Gordello, B. E., Allaminano, P., and Ariza, C.R., (1988). Hypoglycemic effet of Opuntia streplacantha lemaire in non-insulin-dependent diabetes car, 11, Vol : 63-66.
Gouda, A.I, Imorou Toko, I., Salami, S.D., Richert, M., Scippo, M. L., Kestemont, P., Schiffers, B., (2018). Pratiques phytosanitaires et niveau d’exposition aux pesticides des producteurs de coton du nord du Benin. Cah. Agric. 27: 65002.
Heredia, Z.E.A., Bariola, B.J.J. Neuman, J.V., Menta, P.K. (1988). « Improving the moisture resistance of adobe structures ». In: Material and structures 21, 213-221.
John, S., Soloman, P.A., Fasnabi, P.A. (2016). Study on removal of acétamiprid from wastewater by electrocoagulation. Procedia Technology, 24, 616-630.
Lauri., B. (2012). Modelling of pesticide transfers at the scale of watersheds in times of crisis. Ph.D. thésis, Institut National Polytechnique de Toulouse (INPT, Toulouse, France). 160P.
Monkiedje, A., Zuehlke, S., Maniepi, S.J., & Spiteller, M. (2007). Elimination of racemic and enantioebriched Métalaxyl based fungicides Under tropical condition in the field. Chemosphere, 69, 655-663.
Moussa, D., Doubla, A., Kamgang –Youbi, G., & Brisset, J.L. (2007). Post-discharge long life reactive intermediaries involved in the plasma Chemical degradation of an azoic dye. IEEE Transactions on plasma Science, 35, 444-453.
Naïtali, M. Kamgang-Youbi, G., Herry, J. L. (2010). Combined effects of long-living Chemical species during microbial inactivation using atmospheric plasma-treated water. Applied and Environmental Microbiology, 76, 7662-7664.
Njiki, A., Kamgang Youbi, G., Laminsi, S., Lontsi-Djimeli, C., Payom, G., Nola, M., Ngameni, E. (2016). Gliding arc discharge assisted biolodegradation of crystal violet in solution with Aeromonas hydrophila strain. International Journal of Environmental Science and Technology, 13, 263-274. Plasma Chemistry and Plasma Preassing, 22,553-571.
Pimienta-Barrios, E., 1993. Vegetation Cactus (Opuntia). In Underutized Crops: Pulses and Vegetable, p177-191. Ed J. Williams. London, UK. Pimienta-Barrios, E.,1994. Prickly pear (Opuntia Spp) a valuable fruit Crops for the semiarid land de Mexico. J of Arid Env. 28, p1-11.
Pliego, G., Zazo, J.A., Pariente, M.I., Rodriguez, I., Petre, A.L., Leton, P., & Garcia, J. (2014). Treatment of a wastewater from a pesticide manufacture by combined coagulation and Fenton oxidation ESPR (Environmental Science and Pollution Research), 21, 12129-12134.
Rezeg, A. (2004). Removal of hydrolyzed and carboxylated organic acids by coagulation-flocculation with aluminum sulfate. Master’s thesis in Hydraulic Sciences, University of Biskra, Algeria, 81p.
Rodriguez, F.A., and Cantwell, M., 1988. Development Changes in composition and quality of prickly pear Cactus Cladodes (Cactus pearitos). PFHN (Plant Food Human Nutrition). 38, p83-93.
Russel, C.E., and Felker, P. (1987). The prickly pears (Opuntia Spp, Cactacées): A Source of human and amical food in semiarid area. Economic botany, 41(3), 433-445.
Sepulveda, E., and Saenz, C., 1990. Chemical and physical characteristics of prickly pear (Opuntia ficus indica) pulpe. RATA (Revta Agroquin Technol Aliment), 30, p 551-555.
Soulaire, J. (1997). Cactus and Medicine, by LAIGNEL-LAVASTINE. Edition THEBAUT.
Suciu., N.A., Ferrari, T., Ferrari, F., Trevisan, M., & Capri, E., 2011. Pesticide removal from waste spray- tank water by organo Clay adsorption after field application to vineyards. ESPR (Environmental Science and Pollution Research), 18, Vol: 1374-1383.